
Site Management
Our unique site management model is designed to take care of challenges like recruitment, poor site performance/response, quality and compliance issues, etc. Our site management activities are focused on considerably reducing the trial start up time, which overall reduces the trial cost.

Site Identification and Feasibility:-
We have a strong network of pre-assessed sites (experienced as well as new) for efficient conduct of clinical trials. Each site is assessed for infrastructure, qualified professionals, potential subject pool, ethics committee availability & compliance etc. prior to enrolling in our network. We believe in bringing in fresh and unexplored perspective, therefore, we do not hesitate to associate with naïve sites too. We take pride in shaping such sites to optimum levels with our unparalleled site management skills.
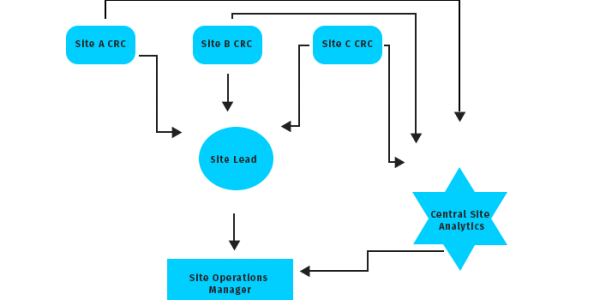
Site Operations:-
It is an evident fact that site CRCs are the backbone of clinical trials; they being the ones coordinating various aspects of the trials, from initiation to closeout. Our trained CRCs are well equipped to take on complex protocols with great ease. They are managed/supervised by highly skilled site leads; who are not only experts in site management, but are also recruitment champions.Please refer to our site opeartions model displayed here:
Central Site Analytics/Centralized Monitoring:-
Our centralized analytics team plays a pivotal role in keeping a vigilant check on site health. Our sites are assessed on multiple parameters to determine the RAG status for each site. Data generated by this team results in proactive risk identification and necessary measures are taken towards mitigation of the same. Site health also assists in identifying additional training needs at the site or any other preventive measures required.
Subject Recruitment and Retention:-
Our champions/experts assist the PI in providing solutions/ideas to upkeep with the recruitment demand. They work closely with the investigator and site staff to identify recruitment challenges (if any) and come up with appropriate resolutions. Our diligent team of CRCs works towards subject retention by meticulously tracking subject visits, enquiring about subject safety and well-being, any hindrances that the subject might be facing, etc. With this, we ensure good subject retention rate and minimal/negligible dropouts.

Ethics Committee Management:-
At ClinPro, we manage and track end to end ethics committee submissions and approvals. We prepare and submit ethics committee submission packages within the TAT specified in the study plans. Our team performs rigorous follow-ups to meet the timelines for EC approvals. We also take care of EC queries and help the sites in resolving them.



